
Mn 2+ = manganese(II) ion Mn 3+ = manganese(III) ion Ni 2+ = nickel(II) ion Ni 3+ = nickel(III) ion Monatomic cations with more than one possible chargeįe 2+ = iron(II) ion Fe 3+ = iron(III) ionĬu + = copper(I) ion Cu 2+ = copper(II) ionĬr 2+ = chromium(II) ionĜr 3+ = chromium(III) ion Other monatomic cations can have more than one possible charge and the charge must be designated by a Roman Numeral in parentheses suffixed to the name of the element. These ions are limited to the Group IA (alkali metals, +1), Group IIA (alkaline metals, +2), zinc (Zn 2+), aluminum (Al 3+), and silver (Ag +). H + = hydrogen ion Mg 2+ = magnesium ion Al 3+ = aluminum ion Monatomic cations with a single possible charge. The first type is named simply by using the element name followed by the separate word ion. Monoatomic cations fall into two groups those which always occur with a specific charge and those which can possess more than one charge. Note that the zero charge shown in the example above is generally not shown and the absence of an indicated charge means the species is neutral. S 0 + 2 e - Æ S 2- The sulfur atom accepts two additional electrons and attains a charge of -2. If an atom loses electrons, its charge increases by +1 for each electron that is lost or decreases by +1 for each electron that is gained.Īl 0 Æ Al 3+ + 3 e - The aluminum atom has lost three electrons and attained a charge of +3. The charge on an ion is determined by the number of electrons the species has gained or lost. Additionally, both types of ions come in both monatomic (single-atomed) and polyatomic (many atomed) varieties. Cations are atoms, or groups of atoms, which have fewer electrons than came with its atoms and have the corresponding positive charge. Anions are atoms, or groups of atoms, which have more electrons than came with its atoms and thus have an overall negative charge.
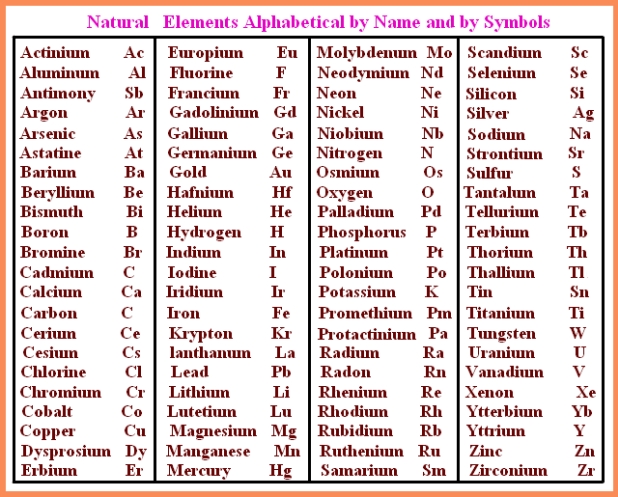
Ions are available in two flavors anions and cations. The number of a particular elements atoms in the molecule is indicated by the subscript to the lower right of the atom.Ĭl 2 = chlorine gas Br 2 = bromine liquid (& vapor) These elements are made up molecules which have two atoms each. For the present discussion, it is only necessary to consider the diatomic ( two-atom) elements. Fortunately, for the vast majority of elements the single-atom ( monatomic) presentation is appropriate, but for a handful of important, common elements it is not. However, when representing an element in an equation it is necessary to use the formula which represents the element as it occurs naturally. They will also appear frequently in the readings, the lectures, and the problems so that with some effort and persistence they will hopefully become more familiar than your neighbors.Īn important aspect of the elements, which may not be obvious, is that the symbol represents a single atom of the element. Learning the names and symbols for the common elements is very much like learning the names of your neighbors: you must memorize them through repetition. Sn = tin (Latin, Stannum) Cu = copper (Latin, Cuperum) W = tungsten (German, Wolfram) Sb = antimony (Latin, Stibnum) K = potassium (Latin, Kalium) Pb = lead (Latin, Plumbum) Na = sodium (Latin, Natrium) Ag = silver (Latin, Argentum)
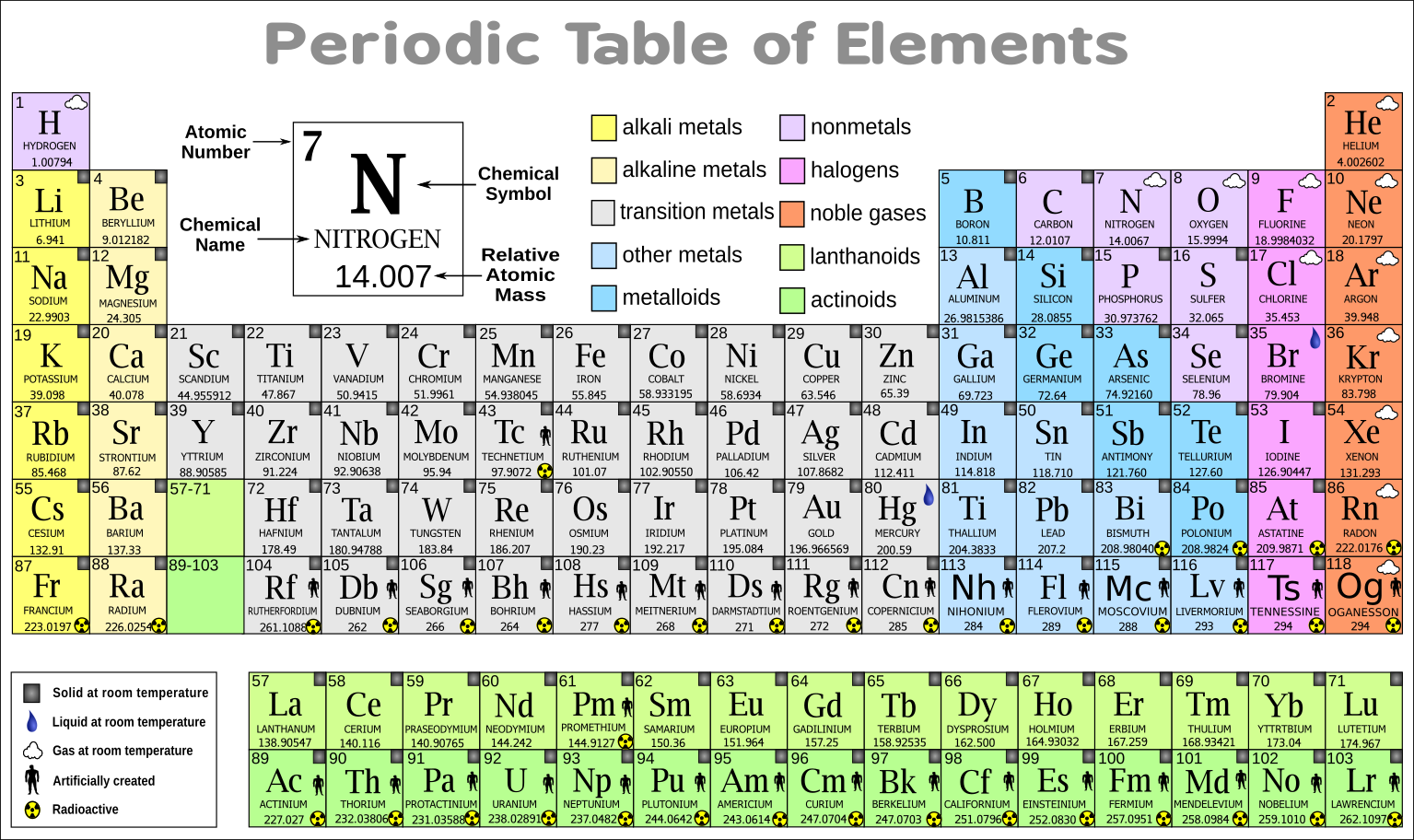
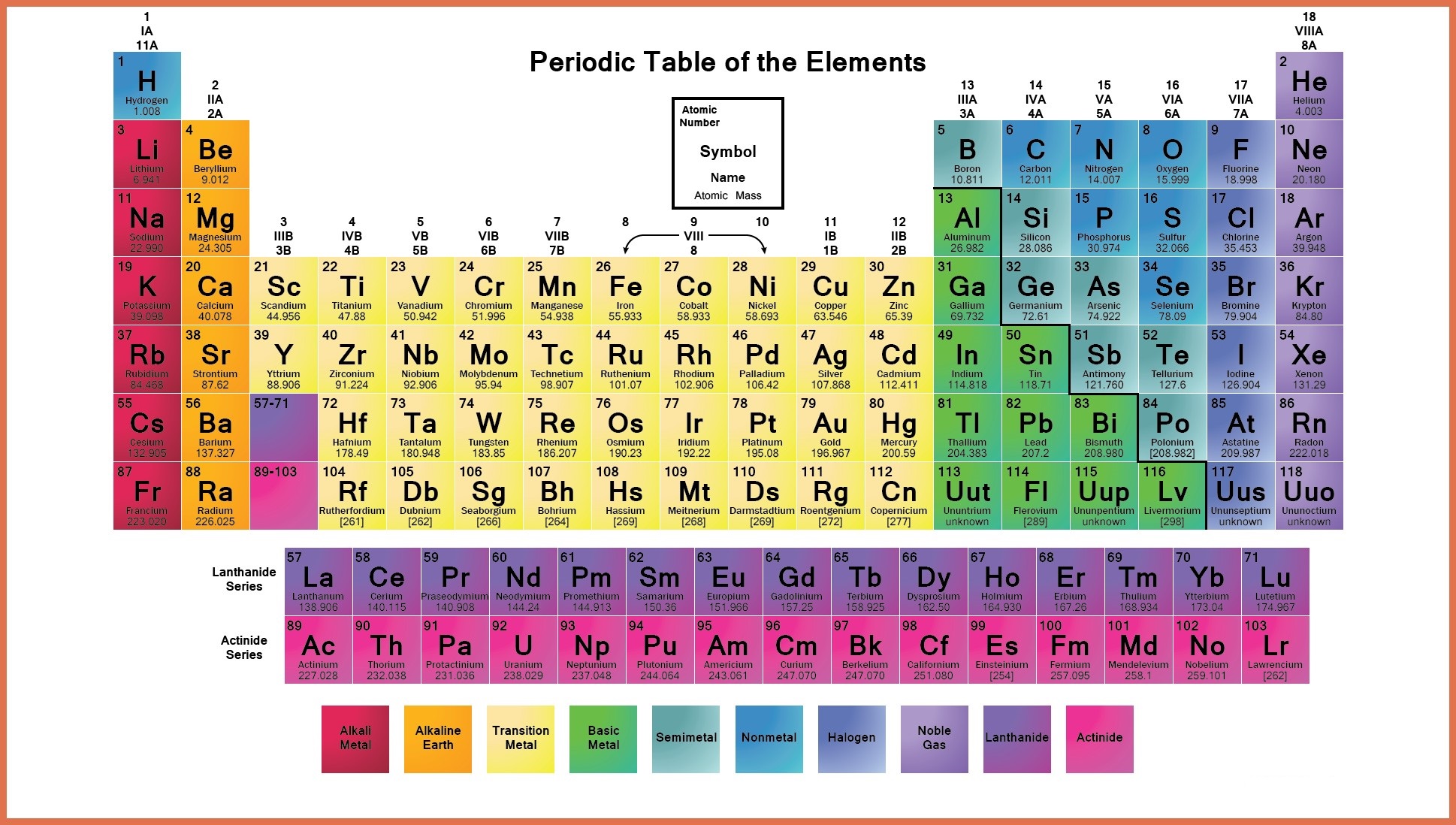
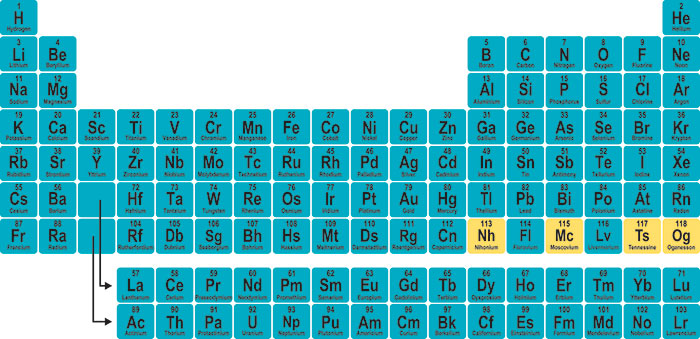
The symbol is not always an abbreviation of the elements English name: the symbol is sometimes an abbreviation of the elements name in another language such as Latin. All symbols consist of one or two letters with the first letter ALWAYS being UPPER CASE and the second letter, if required, is always lower case. Each element has been assigned a name and a symbol, which in this case are oxygen and O, respectively. Atomic mass numbers and atomic numbers for the elements will not be discussed here, but their positions are shown for reinforcement. The example shown is of the element oxygen. Elements are represented as shown in the figure below: With this in mind, it is appropriate that the simplest case be examined first. However, it must be remembered that even very simple can be very difficult when the basics are not understood. This shorthand is systematic and, for most materials, it is simple. Chemists use a form of shorthand to represent the elements, ions, and compounds that we investigate. Sodium - Its Manufacture, Properties, and Uses.Fundamental to the proper understanding and application of the rules of nomenclature is the understanding of the meaning and significance of the symbolism utilized.
#Name of na element pro
Výzkum detekčních trubiček pro bojové chemické látky v České republice.
Comprehensive Treatise on Inorganic and Theoretical Chemistry. Praha: Vysoká škola chemicko-technologická, 2002.


 0 kommentar(er)
0 kommentar(er)
